BCL-2-family protein tBID can act as a BAX-like effector of apoptosis.
Abstract
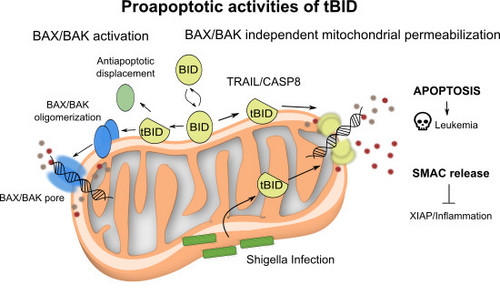
During apoptosis, the BCL-2-family protein tBID promotes mitochondrial permeabilization by activating BAX and BAK and by blocking anti-apoptotic BCL-2 members. Here, we report that tBID can also mediate mitochondrial permeabilization by itself, resulting in release of cytochrome c and mitochondrial DNA, caspase activation and apoptosis even in absence of BAX and BAK. This previously unrecognized activity of tBID depends on helix 6, homologous to the pore-forming regions of BAX and BAK, and can be blocked by pro-survival BCL-2 proteins. Importantly, tBID-mediated mitochondrial permeabilization independent of BAX and BAK is physiologically relevant for SMAC release in the immune response against Shigella infection. Furthermore, it can be exploited to kill leukaemia cells with acquired venetoclax resistance due to lack of active BAX and BAK. Our findings define tBID as an effector of mitochondrial permeabilization in apoptosis and provide a new paradigm for BCL-2 proteins, with implications for anti-bacterial immunity and cancer therapy.
Read more at The EMBO Journal e108690