A common framework of monocyte-derived macrophage activation
Abstract
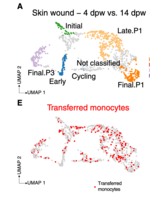
Macrophages populate every organ during homeostasis and disease, displaying features of tissue imprinting and heterogeneous activation. The disconnected picture of macrophage biology that has emerged from these observations is a barrier for integration across models or with in vitro macrophage activation paradigms. We set out to contextualize macrophage heterogeneity across mouse tissues and inflammatory conditions, specifically aiming to define a common framework of macrophage activation. We built a predictive model with which we mapped the activation of macrophages across 12 tissues and 25 biological conditions, finding a notable commonality and finite number of transcriptional profiles, in particular among infiltrating macrophages, which we modeled as defined stages along four conserved activation paths. These activation paths include a "phagocytic" regulatory path, an "inflammatory" cytokine-producing path, an "oxidative stress" antimicrobial path, or a "remodeling" extracellular matrix deposition path. We verified this model with adoptive cell transfer experiments and identified transient RELMɑ expression as a feature of monocyte-derived macrophage tissue engraftment. We propose that this integrative approach of macrophage classification allows the establishment of a common predictive framework of monocyte-derived macrophage activation in inflammation and homeostasis.
Read more at Science Immunology 7, eabl7482